Principle and functioning
Positive resist
The addition of photoactive compounds (PACs, naphthoquinone diazide (NQD)) to alkali-soluble novolacs reduces the alkali-solubility of resists films. The alkali-soluble OH-groups of the novolacs are blocked by NQDs (inhibitory effect) so that alkaline developer is not able to attack. After an exposure in the UV-range (308 – 450 nm) using an exposure mask, the light-sensitive compound reacts to the respective indene carboxylic acid derivative which increases the alkali-solubility for positive resists by a factor of approximately 100 (see Fig. 1). After development, only those areas which were protected by the mask will remain, while exposed areas are removed.
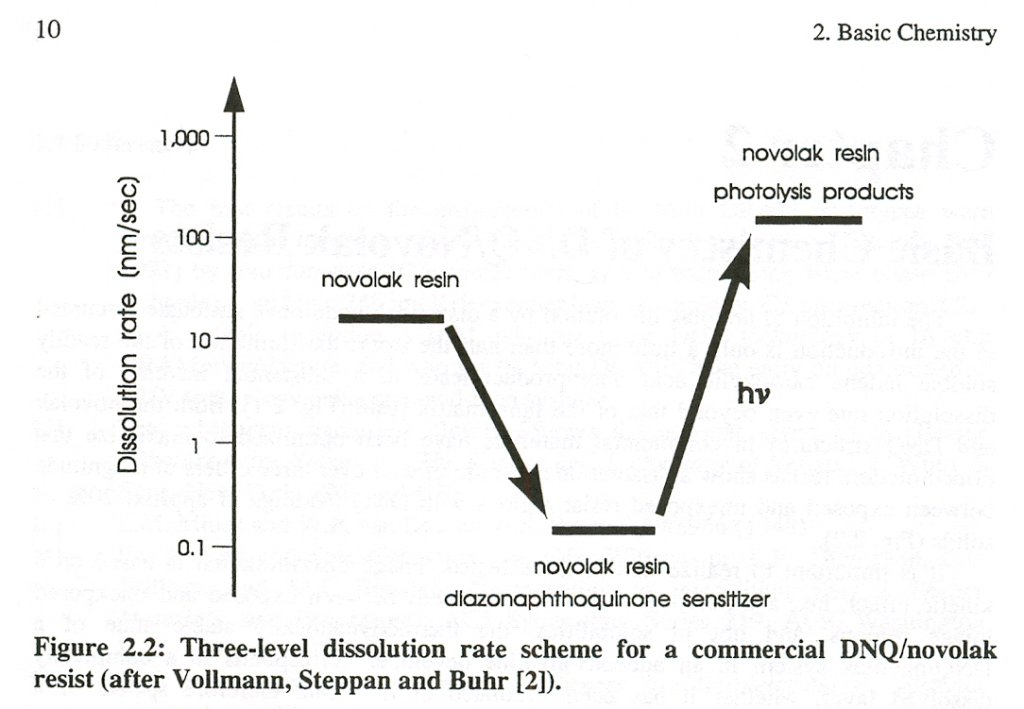
Fig. 1, reference: „Diazonaphthoquinone-based Resists“ by Ralph Dammel, page 10
The development speed of exposed and unexposed areas depends on the PAC-content. With increasing PAC concentration, the dissolution rate of unexposed areas is successively reduced due to a higher concentration of alkali-soluble indene carboxylic acids (see Fig. 2). To obtain maximum sensitivity it is sufficient to expose approx. 30 – 40 % of PACs in a film. A complete exposure requires only additional light energy, while the dissolution rate is only marginally increased. The inhibitory effect is already more or less compensated at 40 % exposure.
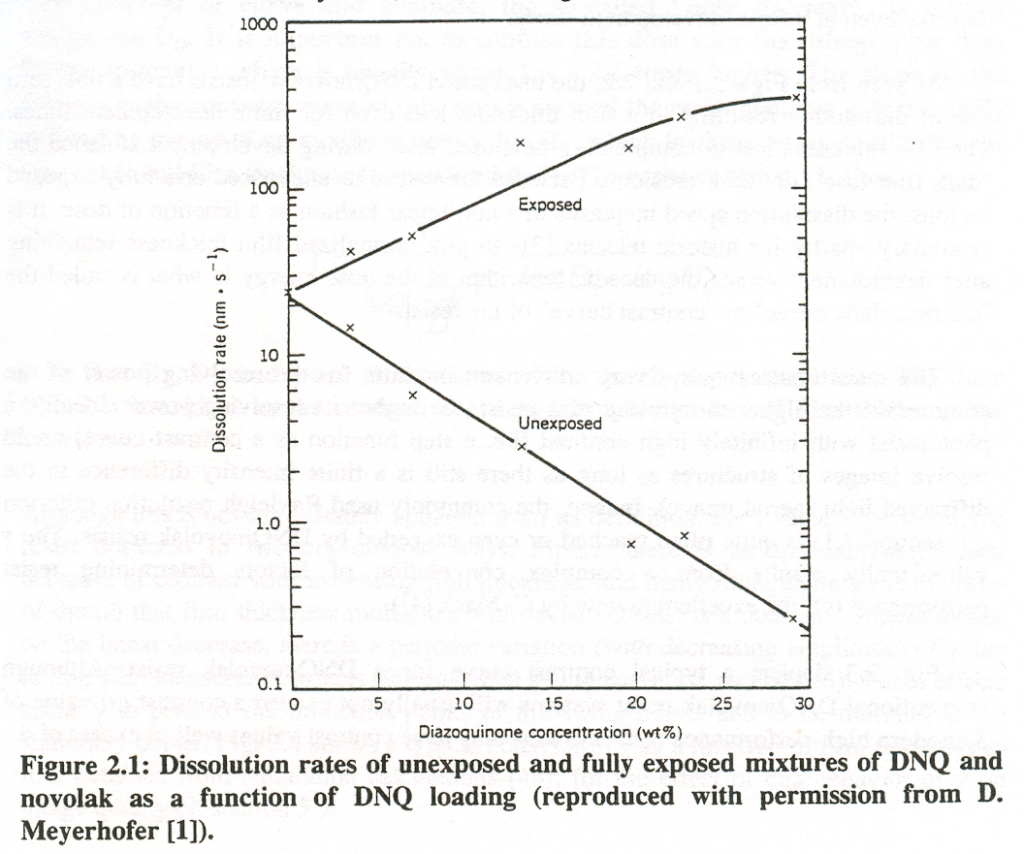
Fig. 2, reference: „Diazonaphthoquinone-based Resists“ by Ralph Dammel, page 9
Negative resist
The cross-linkers bisazide, acidifiers and aminic compounds influence the alkali- solubility of novolacs only marginally. Since cross-linkers are almost exclusively non-soluble under alkaline conditions, the solubility of the negative resist is only slightly decreased in the presence of cross-linkers as compared to the pure novolac. An exposure with subsequent bake step leads to cross-linking of the exposed negative resist areas. For chemically enhanced resists (e.g. AR-N 4400), the bake step after exposure is absolutely essential since cross-linking only occurs under these conditions. Cross-linking of radical cross-linkers like bisazide (e.g. AR-N 4240) however occurs already at room temperature. An additional bake after exposure may result in a higher sensitivity if radical cross-linkers are used.
A cross-linking step renders exposed areas insoluble. These areas are consequently not removed during development. Unexposed areas however remain soluble and are removed by the developer.
Overview of basic chemistry
forays through the lithography of microelectronics
principle and functioning